Month: februar 2016
Climate change in the Himalayas

The Himalayas are the “third Pole” of the Earth, and form the largest body of ice outside the polar regions. They are the source of fresh water for a large number of rivers that flow across the Indo-Gangetic plains. The Himalayan glaciers support perennial rivers such as the Indus, Ganga and Brahmaputra, which support millions of people in South Asian countries (Pakistan, Nepal, Bhutan, India and Bangladesh). Therefore, any catastrophic change in the mountain ecosystem directly affects the lives of the people who live here, who depend directly or indirectly on the waterbodies in the mountains.
The Intergovernmental Panel on Climate Change (IPCC), claims that many Himalayan glaciers are retreating faster than in any other part of the world. However, the extent to which the Himalayan glaciers are expected to melt is uncertain.It is likely that only small glaciers will disappear in the near future, whereas large glaciers located above 5500-6000m will not disappear in this century (Vetaas 2007).
Many studies suggest that warming in the Himalayas has exceeded the global average of 0.74°C over the last 100 years (Solomon et al 2007).The rise in temperature threatens the mountain ecosystem (Shrestha et al. 1999; Nogues-Bravo et al. 2007; Colwell et al. 2008). However, mountain regions are characterized by a complex topography, which helps to provide a spatial buffer for climate change (Peterson et al. 1997). Thus, a small change in temperature does not have a huge impact on the mountain ecosystem, since a short shift uphill can be sufficient to buffer small rise in temperature(Sandel et al. 2011). Due to this topographic effect, the spatial velocity of temperature change is very high in mountain biomes of lower latitudes in comparison to plateaus at higher latitudes (Loarie et al. 2009).
The ranges of plants and animals are moving in response to recent climate change (Parmesan and Yohe 2003), and the dispersal limits of these species determines their vulnerability to local extinction (Sandel et al 2011). Species that have high dispersal capacities are less vulnerable to climate change than species with weak dispersal capacities (Loarie et al. 2009;Sandel et al. 2011).Thus, plateaus ,which are supposed to be most vulnerable to climate change tend to have species which have high dispersal capacities, whereas species which have low dispersal capacities remain in the mountains (Sandel et al. 2011).
Climate change velocity is a measure of the local rate of displacement of climatic condition over the Earth’s surface (Loarie et al 200), and is calculated by dividing the rate of climate change through time by the local rate of climate change across space (Sandel et al 2011). It has been demonstrated that the regions with the highest spatial velocity of climate change have more endemic species, and the regions with a lower spatial velocity of climate change have few endemic species(Sandel et al. 2011). Thus, mountains which have a high spatial climate velocity, support the most endemic species on earth.
The Himalayan Mountains, are likely to experience extensive changes in the environment, biodiversity, and socioeconomic conditions. Land use and land cover changes are probably the reason behind the recent changes in ecosystem functioning in the mountains (Munsi et al. 2010).The rates of loss and fragmentation in Himalayan forests pose a great threat to global biodiversity (Nautiyal and Kaechela 2007). Land use and land cover changes are the most important and easily measurable indicators of global ecological change (Lambin et al. 2001; Di Gregorio 2005). They have a direct impact on mountain biological diversity (Sala et al. 2000), which contributes to local, regional, and global climate change (Chase et al. 1999; Houghton et al. 1999). Land use and land cover change may cause land degradation by altering ecosystem services and livelihood support systems that affect the vulnerability of people (Sharma et al. 2009).
People living in the mountains are highly affected by the impact of extreme weather events. However, most of the people living in the developing world have no idea about the severe impact of long-term climate change in the mountain regions (Sharma et al. 2009). They have faced disasters all year around, including floods, increasing temperatures, storms, rainfall in the high mountains, irregular rainfall and drought. The Seti Flood in Kaski, Nepal ( in 2012) is just one example. The massive Ice-landslide on Annapurna IV (7525m.) blocked the gorge of the Seti river, and caused the devastating flood that killed 72 people and swept away various settlements (IUCN 2012).
The effects of global change in the Himalayas could ruin thousands of lives in the region, however, only few detailed studies have been carried out on observed land use and land cover change (Nautiyal and Kaechela 2007), as well as climate change (Solomon et al. 2007; Nogues-Bravo et al. 2007), and any generalizations that have been made from those could be challenging in making policies. Although the global community is currently trying to understand the effects of climate change on mountain ecosystems, a huge information gap needs to be overcome to understand Himalayan ecosystem functioning, and to maintain ecosystem resilience.
References
Chase, T.N., PielkeSr, R.A., Kittel, T.G.F., Nemani, R.R. and Running, S.W., 2000. Simulated impacts of historical land cover changes on global climate in northern winter. Climate Dynamics, 16(2-3), pp.93-105.
Colwell, R. K., Brehm, G., Cardelus, C. L., Gilman, A. C. &Longino, J. T., 2008. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science, 322, 258–261.
Di Gregorio, A., 2005. Land cover classification system: classification concepts and user manual: LCCS (No. 8). Food &Agriculture Org.
Houghton, R.A., Hackler, J.L. and Lawrence, K.T., 1999. The US carbon budget: contributions from land-use change. Science, 285(5427), pp.574-578.
IUCN., 2012. The Severe Impact of Climate Change in Himalayas.http://www.iucn.org/media/media_awards/?10268
Lambin, E.F., Turner, B.L., Geist, H.J., Agbola, S.B., Angelsen, A., Bruce, J.W., Coomes, O.T., Dirzo, R., Fischer, G., Folke, C. and George, P.S., 2001. The causes of land-use and land-cover change: moving beyond the myths. Global environmental change, 11(4), pp.261-269.
Loarie, S.R., Duffy, P.B., Hamilton, H., Asner, G.P., Field, C.B. and Ackerly, D.D., 2009. The velocityofclimate change. Nature, 462(7276), pp.1052-1055.
Munsi, M., Malaviya, S., Oinam, G. and Joshi, P.K., 2010.A landscape approach for quantifying land-use and land-cover change (1976–2006) in middle Himalaya. Regional Environmental Change, 10(2), pp.145-155.
Nautiyal, S. and Kaechele, H., 2007.Conserving the Himalayan forests: approaches and implications of different conservation regimes. Biodiversity and Conservation, 16(13), pp.3737-3754.
Nogués-Bravo, D., Araújo, M.B., Errea, M.P. and Martinez-Rica, J.P., 2007.Exposure of global mountain systems to climate warming during the 21st Century. Global Environmental Change, 17(3), pp.420-428.
Vetaas, O.R., 2007. Global Change and its Effect on Glaciers and Cultural Landscape: Historical and Future Considerations. Local Effects of Global Changes in the Himalayas: Manang, Nepal. Kathmandu: Tribhuvan University (Nepal) and University of Bergen (Norway), pp.23-39.
Parmesan, C. and Yohe, G.,2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature, 421, 37–42.
Sala, O.E., Chapin, F.S., Armesto, J.J., Berlow, E., Bloomfield, J., Dirzo, R., Huber-Sanwald, E., Huenneke, L.F., Jackson, R.B., Kinzig, A. and Leemans, R., 2000.Global biodiversity scenarios for the year 2100. Science, 287(5459), pp.1770-1774.
Sandel, B., Arge, L., Dalsgaard, B., Davies, R.G., Gaston, K.J., Sutherland, W.J. and Svenning, J.C., 2011. The influence of Late Quaternary climate-change velocity on species endemism. Science, 334(6056), pp.660-664.
Sharma, E., Chettri, N., Tse-ring, K., Shrestha, AB., Fang Jing., Mool, P. and Eriksson, M., 2009.Climate change impacts and vulnerability in the Eastern Himalayas. Kathmandu: ICIMOD.
Shrestha, A.B., Wake, C.P., Mayewski, P.A. and Dibb, J.E., 1999. Maximum temperature trends in the Himalaya and its vicinity: An analysis based on temperature records from Nepal for the period 1971-94. Journal of climate, 12(9), pp.2775-2786.
Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M.M.H.L. and Miller, H.L., 2007.The physical science basis.Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change, pp.235-337.
By Prakash Bhattarai
Save biodiversity for humanity.
(For the sake of argument)
By Erlend Bersaas.
Increasing temperatures, changes in precipitation patterns, loss of ice, rising sea levels, loss of biodiversity, “the sixth extinction”, freshwater shortage, ocean acidification. All apparently due to one single species astonishing evolutionary success. Homo sapiens sapiens. Humans.
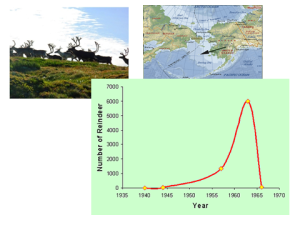
The impact our species have had is so vast that we are in the process of defining our own epoch. The Anthropocene (Vaughan, 2016). The ongoing debate on when the Anthropocene started is not that interesting. The point is that it already has started, and we need to slow down the destruction of our habitat. If not, we are dangerously close to reaching our species carrying capacity. Just feeding an ever-increasing population will eventually become difficult should there not come a second “green revolution” (Godfray et al. 2010). We do not want to end up like the reindeers of St. Matthews Island, an island in the Bering Sea, where 29 animals were introduced in 1944, increased to over 6000, with the result of total collapse by 1966 (Klein 1968).
Hardly any place on the planet is unaffected by humans (Millennium Ecosystem Assessment 2005). Some honourable mentions could be the lakes, which we have not yet disturbed; beneath the cryosphere, but the rest of the planet have experienced some form of human fingerprint. Most of the implications of our behaviour is negative for other species although there are of course exceptions, such as rats and maize, or sheep for that matter, but in general, I do not think too many will disagree with the idea of humans as a negative force on the wildlife and ecosystems. As the Millennium Ecosystem Assessment (2005) states:
Human actions are fundamentally, and to a significant extent irreversibly, changing the diversity of life on Earth, and most of these changes represent a loss of biodiversity. Changes in important components of biological diversity were more rapid in the past 50 years than at any time in human history. Projections and scenarios indicate that these rates will continue, or accelerate, in the future. (Millennium Ecosystem Assessment, 2005, p. 2)
However, why is this a bad thing? Sure, we have transformed a lot of the planet, but in doing so we have become greatly successful. And basically that is what it`s all about.
We are after all competing with every other organism on this planet. The problems arise when we have exhausted our recourses and begin to suffer for it. Thinking that nature is some pristine place, an Eden, where animals and plants live in some sort of stabile harmony, is not going to get us anywhere. We are here, and we are a part of nature as is every other organism. We just dominate everything. We have even made up stories to alleviate us from any moral dilemma concerning other species. The only implications we have had to consider was the one that affected us directly. Just think of what The Bible is saying on the matter of human supremacy on nature:
And God blessed Noah and his sons and said to them, «Be fruitful and multiply, and fill the earth. The fear of you and the terror of you will be on every beast of the earth and on every bird of the sky; with everything that creeps on the ground, and all the fish of the sea, into your hand they are given. Every moving thing that is alive shall be food for you; I give all to you, as I gave the green plant.”
Genesis 9. 1-3 (God, no date.)
The meme of humans as something outside of nature seems to have been greatly successful and have probably participated in our species great evolutionary success. However, because we no longer had to take considerations to what we did with nature, more or less every other organism suffered*.We can argue that this is morally wrong, and that everything has an intrinsic value. It does however look like self-preservation is the underlying argument when trying to convince anyone to save biodiversity and ecosystems.
The UN gives us the argument that we have to save biodiversity because “Biodiversity produces six major services for us: It gives us clean water, food, building materials and medicines. It protects us against extreme weather and help prevent climate change” (Globalis 2016 (my translation)). We can also add different pleasures as recreation, beauty and knowing that some organisms exist, but we should be honest about it. We want to protect life in all its forms due to its value for us. If we do not think anthropocentrically we know what we have to do to stop our domination and destruction of other species, but I for one will not participate in culling half the world’s population.
The Earth is the only world known so far to harbor life. There is nowhere else, at least in the near future, to which our species could migrate. Visit, yes. Settle, not yet. Like it or not, for the moment the Earth is where we make our stand. (Sagan 1994)
*Without going into whether every organism can suffer, because that will only lead to some sort of philosophical argument of where different organisms should be placed on a gradient for the separation of humans from the rest.
Figures:
1: Piraro, D (2007) [Internet] available at: http://bizarro.com/page/2/?s=evolution&submit=Search . Accessed [26.02.2016]
2: Klein, D., Walsh, J. and Shulski, M. (2009) What Killed the Reindeer of Saint Matthew Island? [Internet] available at: http://www.weatherwise.org/Archives/Back Issues/2009/Nov-Dec 2009/full-Reindeer.html. Graph from: [ Internet]http://qualicuminstitute.ca/tenets/317-2/ . Accessed [25.02.2016]
3: Hardin (No Date) [Internet] available at: http://thechive.com/2009/02/26/every-single-evolution-cartoon-we-could-get-our-hands-on-36-photos/ . Accessed [26.02.2016]
4: NASA (2015) Blue Marble – Image of the Earth from Apollo 17. [Internet] http://www.nasa.gov/content/blue-marble-image-of-the-earth-from-apollo-17 . Accessed [26.02.2016]
Sources:
Globalis (2016) Hvorfor er naturmangfold viktig? [Internet] http://www.globalis.no/Tema/Naturmangfold/2.-Hvorfor-er-naturmangfold-viktig .Accessed [25.02.2016]
God (No Date) The Bible. Nicaea.
Godfray H.C.J., J. R. Beddington, I.R. Crute, L. Haddad, D. Lawrence, J.F. Muir, J. Pretty, S.
Robinson, S. M. Thomas, C. Toulmin (2010) “Food: Security: The Challenge of
Feeding 9 Billion People”. Science. 327: 812-818
Klein, D. R. (1968) The introduction, increase, and crash of reindeer on St. Matthew Island.
- Wildl. Manage. 32: 351-367.
Millennium Ecosystem Assessment, 2005. Ecosystems and Human Well-being: Biodiversity Synthesis. World Resources Institute, Washington, DC.
Sagan, C. (1994) Pale blue dot. Also available on YouTube at https://www.youtube.com/watch?v=4PN5JJDh78I
Vaughan, A (2016) Human impact has pushed Earth into the Anthropocene, scientists say. The Guardian. [Internet] http://www.theguardian.com/environment/2016/jan/07/human-impact-has-pushed-earth-into-the-anthropocene-scientists-say . Accessed [25.02.2016]
Creating global food security – Domesticating Poseidon?
By Nickolas James Hawkes
The human population is projected to reach 9.7 billion by 2050 (UN, 2015), falling within most estimates of a global carrying capacity of 8 to 16 billion people (UNEP, 2012); however, an enormous amount of uncertainty exists in these estimates. The uncertainty stems from both natural constraints and the choices we make (Cohen, 1995). This then raises the question: How do we meet the demands of an increasing population? We can increase our food production (a technological solution, Duarte et al., 2009), lower our trophic level (change our diets, Cohen, 1995; Bonhommeau et al., 2013) and (or) ensure a more equitable distribution of resources globally (social solution, Godfray et al. 2010). Furthermore, the problem only increases in complexity when one adds the effects of climate change and resource use into the picture, as the competition for land, water and energy competes with the land available for food production (Godfray et al., 2010).
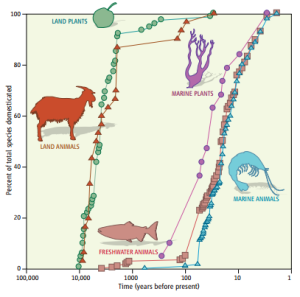
In this blog post, I wish to explore the role of the ocean in providing us food in the future, as the freshwater available for agriculture (directly and indirectly) and the consequences of increased consumption places limits on how many people agriculture can support (Duarte et al. 2009). It is interesting that our domestication of animals and plants, of landscapes and entire ecosystems (Kareiva et al., 2007) is mainly terrestrial, and that our domestication of the ocean only really “began” 100 years ago (figure 1, Duarte, Marbá and Holmer, 2007). Thus, the potential productivity of marine aquaculture – which will henceforth be defined as “mariculture” – and the smaller impact of water limitation (particularly if it is a “closed” system, see Duarte et al. 2009), provide powerful incentives.
Figure 1 (from Duarte, Marbá and Holmer, 2007). The domestication of terrestrial plants and animals began almost 10 000 years ago, whilst the domestication of marine species experienced tremendous growth in the 20th century.
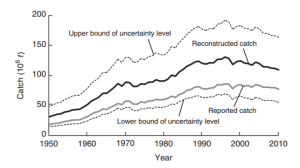
You may wonder why I haven’t discussed fisheries, and the simple answer is that there is not much more “room” in expanding catches (i.e. several stocks are already overexploited, see FAO, 2014). This is reflected in the decreasing level of catches globally (figure 2, Pauly and Zeller, 2016), as well as the increase in the proportion of fisheries production used for direct human consumption (71% in the 1980s and 86% in 2012; FAO, 2014). It has also been estimated that the average recruitment capacity of global fish stocks has declined by 3% of the historical maximum per decade (Britten, Dowd & Worm, 2016). This food source, albeit “unlimited” if fished sustainably, can only support a limited amount of people, in a world of increasing people and demand. Thus a window of opportunity exists for aquaculture and mariculture to provide both food and commodities for humanity.
Figure 2 (from Pauly & Zeller, 2016). Figure showing catch per million tonnes over time with the reported catch (grey line) used in the FAO’s report (2014), and the reconstructed catch (black line). The reconstructed catch includes the reported catch plus estimates of unreported catches.
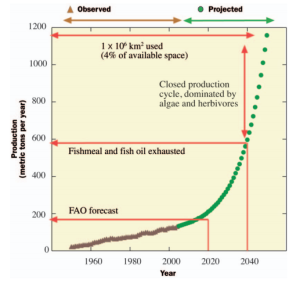
Mariculture has experienced a 10-fold increase in production in 30 years (Duarte et al., 2009), and domestication of both a wider variety of taxa and known species is occurring at rates much higher than terrestrial organisms (Duarte, Marbá and Holmer, 2007). This broader range of taxa has two important implications: One, it allows for polyculture, which can decrease the environmental impact (Neori et al. 2004), and two, it allows for a more diversified market (Duarte, Marbá and Holmer, 2007). What then are its limitations? A parallel in terrestrial agriculture, space, is likely to be one significant limitation in the future (Duarte et al., 2009). As production increases in parallel to demand, increased competition for space is an inevitable outcome. Then comes the challenge of creating closed or “integrated” food systems. This is actually a relatively simple idea, and involves using products from mariculture to feed mariculture (e.g. polyculture, Neori et al., 2004). As an example, if one was to use the maximum possible yield of fishmeal and oil to feed crab and fish mariculture (with the predicted growth rate of 7.5 % per year), production would be limited at 45 to 50 million metric tonnes per year around 2040 (figure 3, Duarte et al., 2009).
Figure 3 (from Duarte et al., 2009). Time-series showing bottlenecks and future challenges of marine food production. Observed values are depicted by brown triangles and predicted values are depicted by green circles. The predicted line is a function of 2005 levels of fish catches and harvest of macroalgal stocks, as well as mariculture’s current growth rate of 7.5% per year.
The future of mariculture certainly looks very bright, and may be the next food revolution in human history (Duarte et al., 2009). With that said, a parallel in agriculture, intensification, can bring increased risks of diseases, species introductions, loss of biodiversity and altered ecosystem services (Duarte, Marbá and Holmer, 2007; Béné et al., 2016). Thus, a sustainable model that optimises yield whilst minimising environmental impacts will be the way forward. I must also add that whilst technological solutions (e.g. polycultures and vertical farming) will aid in creating global food security, there is no single long-term solution. If productivity in food production does not “keep up” with an increasing population with increasing demands, pressure to seek short-term solutions will increase, potentially degrading the largest common of all (tragedy of the commons, Hardin, 1968). If we are to ensure sustainable and equitable food security for all, a multifaceted strategy is needed (Godfray et al., 2010).
References
- Béné, C., Arthur, R., Norbury, H., Allison, E. H., Beveridge, M., Bush, S., Campling, L., Leschen, W., Little, D., Squires, D., Thilsted, S. H., Troell, M. and Williams, M. (2016) Contribution of Fisheries and Aquaculture to Food Security and Poverty Reduction: Assessing the Current Evidence. World Development, 79, pp. 177-196.
- Bonhommeau, S., Dubroca, L., Le Pape, O., Barde, J., Kaplan, D. M., Chassot, E. and Nieblas, A. E. (2013) Eating up the world’s food web and the human trophic level. Proc Natl Acad Sci U S A, 110(51), pp. 20617-20.
- Britten, G. L., Dowd, M. and Worm, B. (2016) Changing recruitment capacity in global fish stocks. Proc Natl Acad Sci U S A, 113(1), pp. 134-9.
- Cohen, J. E. (1995) Population growth and earth’s human carrying capacity. Science, 269(5222), pp. 341-6.
- Duarte, C. M., Holmer, M., Olsen, Y., Soto, D., Marba, N., Guiu, J., Black, K. and Karakassis, I. (2009) Will the Oceans Help Feed Humanity? . BioScience, 59, pp. 967-76.
- Duarte, C. M., Marba, N. and Holmer, M. (2007) Ecology. Rapid domestication of marine species. Science, 316(5823), pp. 382-3.
- FAO (2014) The State of World Fisheries and Aquaculture 2014. in,Rome.
- Godfray, H. C., Beddington, J. R., Crute, I. R., Haddad, L., Lawrence, D., Muir, J. F., Pretty, J., Robinson, S., Thomas, S. M. and Toulmin, C. (2010) Food security: the challenge of feeding 9 billion people. Science, 327(5967), pp. 812-8.
- Hardin, G. (1968) The Tragedy of the Commons. Science, 162(3859), pp. 1243-8.
- Kareiva, P., Watts, S., McDonald, R. and Boucher, T. (2007) Domesticated nature: shaping landscapes and ecosystems for human welfare. Science, 316(5833), pp. 1866-9.
- Neori, A., Chopin, T., Troell, M., Buschmann, A. H., Kraemer, G. P., Halling, C., Shpigel, M. and Yarish, C. (2004) Integrated aquaculture: rationale, evolution and state of the art emphasizing seweed biofiltration in modern mariculture. Aquaculture, 231, pp. 361-391.
- Pauly, D. and Zeller, D. (2016) Catch reconstructions reveal that global marine fisheries catches are higher than reported and declining. Nat Commun, 7, pp. 10244.
- United Nations Department of Economic and Social Affaris / Population Division. (2015) World Population Prospects. New York: United Nations.
- United Nations Environment Programme. Global Environmental Alert Service (GEAS). (2012) One Planet, How Many People? A Review of Earth’s Carrying Capacity.
The Climate: we are changing it, and we can change it again
By Hanna Sundahl
You have probably seen by now pictures of smog engulfing major cities in China, and the pictures of burning forests in Indonesia last year. What do these have in common? They are large-scale human-induced (anthropogenic) changes. The former is attributed to the large amounts of coal produced for the Chinese energy sector (Bloomberg, 2015). The latter scenario is occurring because of a combination of predictable and unpredictable causes: large tracts of forest are burned to make way for large-scale production of e.g. oil palms (used for cooking oil, snacks, and cosmetics, among other things), but the periodic, unpredictable climate event El Niño we are experiencing right now also increases drought across the peninsula, reducing the amount of rain that otherwise extinguishes these fires (Balch, 2015).
But while this should show you at what scales we can change the planet, climate skeptics will argue that global climate change is entirely natural, not anthropogenic. Climate change does indeed occur continuously, and we can observe changes until around 400,000 years ago by looking at the gas percentages in trapped air bubbles in ice cores such as those in Antarctica (Barnola, et al., 2003). But evidence from this as well as from present-day observations like those made at the Mauna Loa volcano in Hawaii show atmospheric CO2 content and the average global temperature have been on a growing trend ever since the start of the Industrial Revolution in the 1800s, and the last decade has the been the warmest on record, see figure below (IPCC, 2014). By now, most climate scientists (97%) agree that we are seeing global warming and that this can largely be attributed to anthropogenic fossil fuel burning and land-use change (NASA, 2016). But it’s not entirely the amount of CO2 we’re releasing, but the rate that should make you turn off your TV, hop off your couch, and run over to that climate march. Digging up and burning fossils that have been sequestered over some million years disrupts the slow natural carbon cycle (IPCC, 2014). Now, considering humans have been so adept at extracting all this in such a short time, it makes sense to think that the earth is “fighting back”, trying to reach homeostasis much like a living organism.
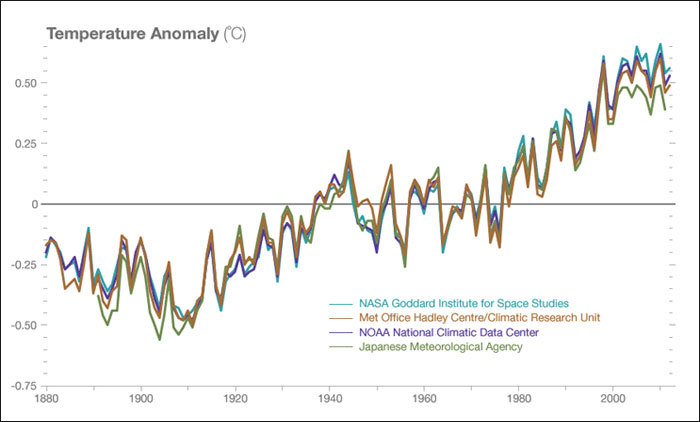
Temperature data from four international science institutions, showing an increasing trend and record warmth for the past decade. Data sources: NASA’s Goddard Institute for Space Studies, NOAA National Climatic Data Center, Met Office Hadley Centre/Climatic Research Unit and the Japanese Meteorological Agency. Image © NASA. Retrieved from: http://climate.nasa.gov/scientific-consensus/
Climate change affects us in all sorts of ways: extreme weather events increase in frequency, food security and shelter become threatened, which in turn worsens poverty and health, and destabilizes social and political infrastructures. But to fight against this, we have to be frank about what the solution is: stop using fossil fuels and start using renewable, cleaner energy. Our reluctance to switch to more renewable energy sources exists in part due to misinformation. For example, solar panel and wind turbine production are actually declining in cost, and, once installed, are essentially free, unlike plantations running on fossil fuels. Production is continuously increasing, including in Saudi Arabia and China, the latter mainly to deal with the health-damaging smog problem (Lewis, 2015). As the movie “This Changes Everything” states, perhaps the status quo Capitalism is a major source of this evil?
Al Gore sums up the motivation to fight against climate change more succinctly than I can in one blog post with his newest TED talk “The case for optimism on climate change”, which I urge you all to see → http://tinyurl.com/hpd4yxp. He lists all the challenges that I mentioned and more, but also brings to light the motivating fact that we CAN change and ARE in fact heading in the right direction to a more renewable, sustainable energy regime.
So it’s not actually the lack of investment or knowledge holding us back, though public outreach can always be improved. As cheesy as it sounds, we just need to be able to BELIEVE it is possible to slow down anthropogenic climate change.
Perhaps it’s alright for some to think we will become the next dinosaurs anyway, let’s just go about with business as usual…but, why hasten the extinction process? I don’t believe it’s a time to give up; consider instead the optimism in seeing the combined forces of people making their voices heard together with research and investment made into more sustainable solutions for the future.
References
Balch, O. (2015, November 11). Indonesia’s forest fires: everything you need to know. Retrieved from Guardian sustainable business: http://www.theguardian.com/sustainable-business/2015/nov/11/indonesia-forest-fires-explained-haze-palm-oil-timber-burning
Barnola, J. M., Raynaud, D., Lorius, C., & Barkov, N. I. (2003). Historical CO2 record from the Vostok ice core. Trends: A Compendium of Data on Global Change. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, U.S. Department of Energy, Oak Ridge, Tennessee, U.S.A.
Bloomberg. (2015, December 30). China to Halt New Coal Mine Approvals Amid Pollution Fight. Retrieved from Bloomberg Business: http://www.bloomberg.com/news/articles/2015-12-30/china-to-suspend-new-coal-mine-approvals-amid-pollution-fight
IPCC. (2014). Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, R.K. Pachauri and L.A. Meyer (eds.)]. Geneva: IPCC.
Lewis, A. (Director). (2015). This Changes Everything by Naomi Klein [Motion Picture].
NASA. (2016). Scientific consensus: Earth’s climate is warming. Retrieved from Global Climate Change: Vital Signs of the Planet: http://climate.nasa.gov/scientific-consensus/
Why we should care more about our coasts: coral reefs, mangroves and seagrass meadows in the tropics
By Hanna Sundahl
When you think of a tropical ocean, you probably think of pristine, turquoise waters, with colorful reefs spread below the surface teeming with fish and other stunning marine life. Indeed, this is what a healthy tropical coral reef should look like. Even though coral reefs cover less than 0.1% of the ocean surface area, they sure pack a punch in terms of biodiversity, with vertebrate species densities far greater than those found in rainforests (Kaiser, et al., 2011).

The coral reef at Heron Island, Great Barrier Reef, in all its splendor. (c) Sarah Norris, Published in Voyeur January 2014. Retrieved from: https://travel.virginaustralia.com/nz/voyeur/heavenly-heron-island
Coral reefs are a vital habitat for a number of reasons. Not only do they provide food, shelter and breeding grounds for marine life, reefs are also important for us people, since not only do they give us food (in many areas fish is the main source of protein), they also slow down wave action and serve as inexpensive coastal protection (Hoegh-Guldberg, et al., 2007). Coral reefs also have the potential to replace rainforests as the new “medicine cabinet” of nature to provide natural chemicals that can treat all sorts of human ailments (National Oceanic and Atmospheric Administration). And of course, they attract millions of tourists coming to dive and snorkel, making them a very important source of income for coastal regions (IPCC, 2014).
However, coral reefs are only part of the picture. They also have less well-known “sister ecosystems”, namely the muddy-looking mangrove forests and the seemingly simple-looking seagrass meadows. These habitats provide essential nursery spots for the juveniles of many of the fish we see while diving in coral reefs, including sweetlips, snappers, and surgeonfish (our beloved Dory) (Nagelkerken, et al., 2000). With their complex root systems keeping sediments in place, mangroves and seagrasses also help serve as coastal protection (Kaiser, et al., 2011).

Mangrove forest in Papua, Indonesia. (c) Photo credit: Ethan Daniels. Retrieved from: http://blog.nature.org/science/2013/10/11/new-science-mangrove-forests-carbon-store-map/

Seagrass meadow with predatory fish lurking above. (c) NOAA, Heather Dine. Retrieved from: http://www.amnh.org/education/resources/rfl/web/oceanguide/key.html
These three connected habitats are havens of biodiversity, sources of food and security for people, and all of them are unfortunately threatened by human coastal development. Mangroves and seagrass meadows suffer from chemical runoffs from shrimp aquaculture and farming nearby, as well as from outright mechanical destruction for land reclamation (American Museum of Natural History). Coral reefs also suffer from farmland fertilizer runoff that can cause eutrophication, leading to a habitat dominated by fast-growing algae rather than coral (Hoegh-Guldberg, et al., 2007). Careless tourists kicking and removing corals and associated living organisms, and irresponsible fishing practices like dynamite and cyanide fishing, add further pressures to these fragile ecosystems.
Considering their importance and vulnerability, protective measures need to be taken for these habitats. One such measure is to create strictly regulated Marine Protected Areas (MPAs), where limited or no human development can occur, and where visitors have to be extremely careful and considerate (National Oceanic and Atmospheric Administration). Such protected areas act as a refuge, a safe space in which biodiversity thrives, and act as a source population for re-colonization of surrounding habitat. Top priority areas are those with high endemism, where the habitat is still very intact, or that are important for feeding and breeding of long-distance migrators like manta rays or whales. The cost of maintaining MPAs is outweighed by their increased attractiveness for responsible divers and tourists in love with these healthy marine habitats.
MPAs, together with other regulations such as fishing quotas, can allow future generations to acquire the protein they need from the sea, live at the coast, and thrive on responsible tourism. Of course, there are global threats that cannot be kept out of these parks – notably ocean warming and acidification – which is why we also need to pay attention to our carbon emissions problem, the topic of my next post. But with this, I, as a diver and marine biologist, urge you to be a responsible tourist and citizen of the world and care about these fragile, yet incredibly important habitats.
References
American Museum of Natural History. (n.d.). Mangrove Threats and Solutions. Retrieved from Mangroves: the Roots of the Sea: http://www.amnh.org/explore/science-bulletins/bio/documentaries/mangroves-the-roots-of-the-sea/mangrove-threats-and-solutions
Hoegh-Guldberg, O., et al. (2007). Coral Reefs Under Rapid Climate Change and Ocean Acidification. Science, 318(1737), 1737-1742.
IPCC. (2014). Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, R.K. Pachauri and L.A. Meyer (eds.)]. Geneva: IPCC.
Kaiser, M. J., et al. (2011). Mangrove Forests and Seagrass Meadows. In Marine Ecology: processes, systems, and impacts (2nd Ed) (pp. 277-303). New York: Oxford University Press.
Kaiser, M. J., et al. (2011). Coral Reefs. In Marine Ecology: processes, systems, and impacts (2nd Ed) (pp. 305-323). New York: Oxford University Press.
Nagelkerken, I., et al. (2000). Importance of Mangroves, Seagrass Beds and the Shallow Coral Reef as a Nursery for Important Coral Reef Fishes, Using a Visual Census Technique. Estuarine, Coastal and Shelf Science, 31-44.
National Oceanic and Atmospheric Administration. Marine Protected Areas. Retrieved from http://oceanservice.noaa.gov/ecosystems/mpa/
National Oceanic and Atmospheric Administration. Medicine. Retrieved from NOAA Coral Reef Conservation Program: http://coralreef.noaa.gov/aboutcorals/values/medicine/
Treelines shift
An overview
In Alpine Mountain Environments, when we look from a distance, a kind of boundary line between trees and dwarf shrubs is visible, the fuzzy line or boundary where the trees start to disappear and shrubs and grass become dominant is what ecologists and biogeographers define as a treeline. Ecologists have defined trees as an individual plant which has a height above 2m (in the case of alpine regions) from the ground surface and treeline as the highest elevation of trees in a patch comprising at least three individuals (1). The treeline, in general, is an upper elevational limit of tree growth in mountains. These treelines are physiognomic boundaries along the elevation gradient (2) as a result of heat deficiency, i.e. these are temperature dependent (1). Elevation and latitude are the surrogate of temperature, which indirectly govern the position of treeline (figure 1). Hence, the treelines are sensitive to climate change (3). However, the extent and limit of the treeline ecotone are expected to be governed by several other confounding factors, such as the presence of herbivores, forms of treeline, land-use dynamics, geomorphology, moisture, and species interactions.
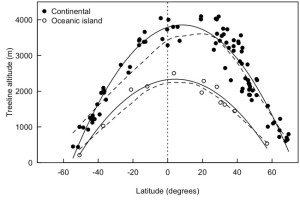
Figure 1: Treeline position around the globe by latitude for continental (filled points) and oceanic island (open points) (4).
In recent decades, there have been many studies focusing on treelines. A “treeline shift” has gained widespread attention, especially amongst vegetation ecologists, biogeographers, and environmentalists over the last few decades. The spatial and temporal span of those studies ranges from local to regional and few years to century. The majority of the small geographic studies have reported the upslope shift of treelines (5, 6); whereas some others found contrasting results (7). Researches have tried to connect the shifts with causative factors. Most of the studies found that the treeline shifts are related to landuse changes, climatic warming and moisture stress, as well as geomorphology, and species interaction.
Theoretically, a warming in climate causes the treeline to move/migrate up-slope in the case of mountains and pole-wards in latitude. This applies directly only in the absence of other interacting factor or their insignificant effect. For instance, the advance of the treeline was found to be a response to the warming in post-little ice age in the Swedish Scandes (8) and there have been several reports from various parts of the world about the treeline shift in response to the recent warming (5, 6). However, the treelines’ response to climatic warming is altered by several factors such as landuse change and disturbance in treeline ecotone.
A global meta-analysis (5) on treeline shifts found that only 52% of treelines have shifted up-slope while about 1.5% of treelines receded which shows that the trend of treeline advance is not universal. In the case of the up-slope shift, a rise in winter temperature the last century is found to be a major driver. The treeline shift is a result of the dispersal and establishment in treeline ecotone, which are highly sensitive to short extreme climatic events such as drought and extreme long clod periods. (9). Extreme events such as drought or severe frosts during growing season affect the recruitment and survival of seedlings, which ultimately affects treeline dynamics (10).
Landuse is another interacting factor that cause treeline shift. It contributes in retreat the treeline (5) or can mask or moderate the upwards shift of the treeline with climatic warming. For instance the treeline in Norway has been controlled by the presence of herbivores (11). Herbivores may consume tree seedlings or destroy them by uprooting, ultimately inhibiting the growth of treeline species in the ecotone. In such cases, once the disturbance is reduced or eliminated from the treeline ecotone, the treelines move towards its climatic limit by shifting the treeline up-slope (12).
To conclude
The response of treelines to global changes is rather complicated when both the climatic warming and landuse acts together. The climatic warming favours treelines advance up-slope while landuse causes the treelines to retreat or stabilize at the same elevation. In such cases, a balanced counteracts of the climatic warming and disturbances conceal the dynamic nature of treelines. However, land abandonment or a reduction in land use intensity may favour treeline up-slope shift. Regardless of the causes of the treeline shift, the shift is site specific and species specific. The response of treeline to climatic warming varies across the phytogeography regions (temperate to the tropical region) (7), and also depends on the form of treeline (5, 10, 13).
In the premise of continued climate change which is projected to continue throughout the 21st century, the treeline is expected to shift further upwards in the altitude and polewards in latitude. The shift may also be influenced by the landuse changes. In the mountains, the up-slope shift of treeline may increase competition and alpine species will suffer from more shade by a new shrub or forest canopy. Similarly, species which are unable to keep pace with warming will lose their suitable habitat affecting their population.
References
1. C. Körner, Alpine plant life: functional plant ecology of high mountain ecosystems. (Springer, Berlin, 2003), pp. XI, 344 s. : ill.
2. U. Schickhoff, in Mountain Ecosystems. Studies in Treeline Ecology, G. Broll, B. Keplin, Eds. (Springer Verlag, Berlin-Heidelberg-New York, 2005), pp. 275-354.
3. C. Körner, J. Paulsen, A world-wide study of high altitude treeline temperatures. J Biogeogr 31, 713-732 (2004).
4. A. Berdanier, Global treeline position. Nature education knowledge 3, 10-11 (2010).
5. M. A. Harsch, P. E. Hulme, M. S. McGlone, R. P. Duncan, Are treelines advancing? A global meta‐analysis of treeline response to climate warming. Ecology letters 12, 1040-1049 (2009).
6. A. Tiwari, Z.-X. Fan, A. S. Jump, S.-F. Li, Z.-K. Zhou, Gradual expansion of moisture sensitive Abies spectabilis forest in the Trans-Himalayan zone of central Nepal associated with climate change. Dendrochronologia, (2016).
7. E. M. Rehm, K. J. Feeley, The inability of tropical cloud forest species to invade grasslands above treeline during climate change: potential explanations and consequences. Ecography 38, 1167-1175 (2015).
8. L. Kullman, L. Öberg, Post‐Little Ice Age tree line rise and climate warming in the Swedish Scandes: a landscape ecological perspective. Journal of Ecology 97, 415-429 (2009).
9. S. T. Gray, J. L. Betancourt, S. T. Jackson, R. G. Eddy, Role of multidecadal climate variability in a range extension of pinyon pine. Ecology 87, 1124-1130 (2006).
10. F.-K. Holtmeier, G. Broll, Treeline advance–driving processes and adverse factors. Landscape Online 1, 1-33 (2007).
11. J. D. Speed, G. Austrheim, A. J. Hester, A. Mysterud, Experimental evidence for herbivore limitation of the treeline. Ecology 91, 3414-3420 (2010).
12. J. D. M. Speed, G. Austrheim, A. J. Hester, A. Mysterud, Elevational advance of alpine plant communities is buffered by herbivory. J Veg Sci 23, 617-625 (2012).
13. U. Schickhoff et al., Do Himalayan treelines respond to recent climate change? An evaluation of sensitivity indicators. Earth System Dynamics 6, 245 (2015).
An Argument for Managing the World’s Meat Consumption
Some of the greatest concerns of our time is the overuse of resources, global warming and the great increase in the human population. In spite of that 50% of the Earth’s terrestrial surface is used for crop-production and grazing areas (Kareiva et al. 2007) we will not be able to feed our soon to be 9 billion people ( Godfray et al. 2010). In addition to this, it is in human interest to reduce the land used for agriculture due to exhaustion and conservation of areas. It is important that we start using our resources more efficiently, and in a more sustainable way before it is too late.
Around 30% of the Earth’s surface, or 80% of all land used by humans, is used to support livestock for meat production (Stehfest et al. 2009). A big part of this could be used to create food on a lower tropical level, which means more energy (food) on the same amount of area, which could in turn feed more people. Only about 10 percent of the energy stored in the primary producers (greens) is transferred to the consumer. Not to forget- meat production is one of our biggest climate concerns, because of emissions from livestock of methane, nitrous oxide and carbon dioxide, and stands for around 18% of our total greenhouse gas emissions (Stehfest et al. 2009).
To be able to reach a more sustainable way of agriculture, we do not only need a to change the way we do things, but we also need a drastic change in the way the richer part of the world thinks about food (Walsh, 2013). If we were forced to cut down our consumption of meat, it could have major effects on reducing the rate of the global warming, we could sustain the population growth, at least for some time, not to mention the effect it could have on our private health. A more balanced diet than what you often find in the western world today will be economically positive, where many people have a high fat- and sugary diet, and their health issues cost the state a lot of money (Godfray et al. 2010).
This does not mean that we should quit all husbandry and meat production, and that we all should be vegetarians. Meat and other animal products is an important source of proteins and nutrients. In addition, parts of the human livestock are grazing animals who use landscape that could not be used for crop production anyway (Godfray et al. 2010). But it means that it’s time to realize that our earth, which is the only earth we will ever have, needs us to start thinking more long-term, and start using our land so that it can keep feeding us for the years to come. It needs us to realize that we cannot keep consuming in this way, when there is not enough food for the rest of the world.
For every 100 kg of proteins in ruminant meat, we require 60 m2, while beans, peas, lentils and chickpeas only require 25m2 to create the same amount of proteins (Stehfest et al. 2009). This means that we could sustain over twice as many people in proteins by a change in land use. And according to PETA’s (People for the ethical treatment of animals) website, as of February 2016; it takes about 20 times as much land to support a meaty diet, than a fully plant based diet.
The average American eats 122 kg of meat per year; this is 334 grams a day, or 2.3 kg a week (Walsh, 2013). For comparison, the average Norwegian eats 65.4 kg a year (Barclay, 2014), which is just above half the American consumption. The country to score highest on meat consumption per person is Luxembourg with as much as 301.7 kg of meat per year, which equals to 375 grams a day. This is a ridiculous amount of meat, and this amount of beef requires 75m2 of land use per person each day of the year (Barclay, 2014). Of course, the amount of meat is shared between poultry, pork, beef and other meat, where beef requires the biggest amount of land.
Having a meat consumption that would be devastating for the world if adopted by other nations, should be reason enough to manage the meat-culture down to sustainable levels. It is to be expected that developmental countries to increase their meat consumption as they become wealthier (Begon et al. 2014). Reducing the American, and other first world countries’ meat consumption to the levels of India (3.2 kg a year), or Bangladesh (3.6 kg a year) (Barclay, 2014) seems unrealistic, but to reduce it by some, (or even half) should be possible. In fact a declaration called the “Barsac Declaration” were put forth by a group of biologist in 2009, saying that no-one should get more that 35- 40% of their protein from meat, which would reduce the American and other first world countries’ consumption of meat by half. Not only would this do the world so much good in the means of emissions, and the amount of food, but it is also the upper limit recommendation in a healthy diet. (Begon et al. 2014).
By Åsne Brede
References
Kareiva P., S. Watts, R. McDonald, T. Boucher (2007) “ Domesticated Nature: Shaping Landscapes end Ecosystems for Human Welfare” Science 316: 1866-1869.
Stehfest E., L. Bouwman, D.P. van Vurren, M.G.J. den Elzen, B. Eickhout, P.Kabat (2009)
“Climate benefits of changing diet”. Climatic Change. 95:83-102
Godfray H.C.J., J. R. Beddington, I.R. Crute, L. Haddad, D. Lawrence, J.F. Muir, J. Pretty, S.
Robinson, S. M. Thomas, C. Toulmin (2010) “Food: Security: The Challenge of
Feeding 9 Billion People”. Science. 327: 812-818
Walsh B. (2013) “The Triple Whopper Environmental Impact of Global Meat Production”. TIME. Available: http://science.time.com/2013/12/16/the-triple-whopper-environmental-impact-of-global-meat-production. Last accessed 23/02/16
Barclay E. (2014) “A Nation Of Meat Eaters: See How It All Adds Up“. The Salt. Available: http://www.npr.org/sections/thesalt/2012/06/27/155527365/visualizing-a-nation-of-meat-eaters. Last accessed 24/02/16
Begon M., R.W. Howarth, C. R. Townsend. (2014). The ecology of human population growth, disease, and food supply. United States of America: Wiley. p. 406- 440
PETA “Meat and the environment” Available: http://www.peta.org/issues/animals-used-for-food/meat-environment/ Last accessed 25/02/16
Image taken from www.freeimages.com
The sixth mass extinction
By: Ragnhild Gya
Whether we are going into the sixth mass extinction or not has been heavily debated. In 2015 a paper was published by Gerardo Ceballos and his colleagues were they state that the sixth mass extinction is happening, and that we are causing it. I am now going to try and explain their arguments the best I can, you can also read the article yourself (reference in the end of this blog entry).
Extinctions happen all the time. Did you know that over 90 % (and maybe closer to 98%) of all species that has lived on Earth is now extinct? Whether we have a net gain or a net loss of biodiversity and species depends on the rate of occurring new species and the rate of extinctions. This “normal” amount of species going extinct is called the background rate of extinctions. This background rate stands for most of the extinctions in the history of the earth; actually the mass extinctions can only be blamed for 4 % of all extinctions in the last 600 million years.
When the extinction rate is much higher than the background rate, it is classified as a mass extinction. The most famous one is the one that killed the dinosaurs, 65 million years ago, which is called the Cretaceous-Paleogene extinction event. The one that killed the most species is the Permian-Triassic extinction event, which made 95 % of marine species and 70 % of species on land go extinct. Are we also now in a period similar to these two?
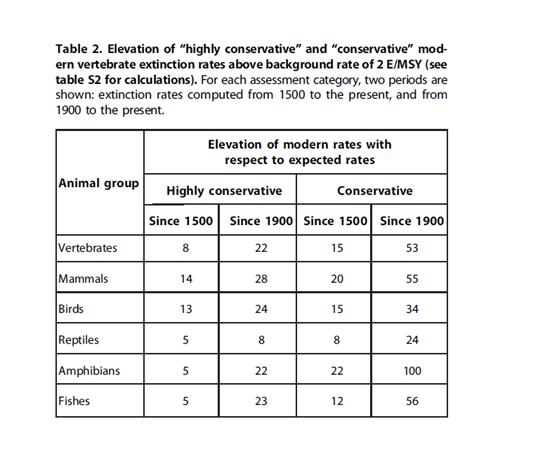
To figure this out scientist estimate the normal background rate of extinctions from fossil records, written observations etc. and compare that to a number that they have calculated for our current extinction rate. A lot of scientists have published papers where they conclude that we are in a mass extinction because of the results of these calculations. Ceballos and his colleagues discussed the fact that the background rates were calculated from marine invertebrates and that the background rate would probably be higher if we considered other groups as well. So instead of using the most used background rates which are between 0.1 and 1 extinction per 10 000 species per 100 years (0.1-1 E/MSY), they used 2 E/MSY. They more than doubled the previous number for the background rate, which should make it harder to get a result that says that we are in the sixth mass extinction if we actually are not.
To find the modern extinction rate, the used the IUCNs (International Union of Conservation of Nature) list over threatened and extinct species. They have three “extinct” categories: “extinct” (EX), “extinct in the wild” (EW) and “possibly extinct” (PE). They calculated using two different approaches: the highly conservative one with only using numbers from the category extinct, and the conservative one using all three categories.
You can see the results of these calculations in the table underneath. The numbers are how much higher the current extinction rate is compared to the background rate. As you can see, it varies from 8 to 100, and this is using the conservative method to calculate it. As a conclusion we are now no longer in doubt about this period – it is the sixth mass extinction.
Table: Ceballos et al. (2015)
Just to remind you what we are losing, here are some species that have gone extinct in the past 100 years:
Extinction 1: The last Thylacine in captivity, pictured above, died in 1936.
Extinction 2: Wester Black Rhino. Once widespread in central west Africa, in 2011 it was declared extinct.
Extinction 3: Golden Toad. Only described to science in 1966, and once abundant in a 30 square mile area of the cloud forest above Monteverde, Costa Rica, none of the toads have been sighted since the 15th of May 1989
References
Kevin J. Gaston and John I. Spicer (2004). Biodiversity – an introduction. 2nd ed. : Blackwell Publishing. p19-48.
Gerardo Ceballos, P. R. Ehrlichm A. D. Barnosky, A. García, R. M. Pringle, T. M. Palmer. (2015). Accelerated modern human-induced species losses: Entering the sixth mass extinction. Science. 1 (5), .
https://en.wikipedia.org/wiki/Extinction_event (24/02/2016)
Pictures:
http://www.treehugger.com/slideshows/natural-sciences/glimpse-what-we-lost-10-extinct-animals-photos/ (24/02/2016)
https://www.flickr.com/photos/martijnmunneke/6521931525/sizes/l/in/photostream/ (24/02/2016)
https://en.wikipedia.org/wiki/File:Bufo_periglenes2.jpg (24/02/2016)
Predicting the consequences of human impact a changing biosphere, implications of the lack of species knowledge.
by Trond R. Oskars
Many species and Biodiversity (1) as a whole is currently under a lot of pressure, not only climate change (2, 3, 4), but also increased direct interference by humans (5, 6, 7, 8, 9). This may lead to extinction events, severe shifts in species interactions and ecosystem composition (10, 11, 12). Due to this assessing current and past biodiversity and understanding their interactions and responses to stressors have become more important to try to predict the outcome of the ongoing human driven change of the biosphere (13, 14).
It is a known problem that there are large gaps in the fossil record (14), the older the rocks the bigger the chance that older fossils will not survive to be discovered and cannot be expected to match the diversity represented in more recent fossil assemblages (14, 15, 16). However the fossil record can still tell us a lot about long time stability of organism groups and ecosystems (14, 16), thus gives us the board picture of the effect of stressors comparable to human actions. However for a more detailed view we need data from living species (14).
Here we encounter an additional problem the lack of knowledge of extant species of many groups. About 1.2 to 1.9 million species have been formally described and given a name (17, 18). The number of species that currently exist in the world (not counting bacteria and their kin) varies by the method used, but is has been estimated as high as 8.7 million (18, 19). This leaves the majority (over 80%) of all species unknown or unnamed (20).
This opens for the possibility that species may fail to be recognized, discovered or described before being driven to extinction (13, 18). Even for relatively well known ecosystems (tropical forests) and well-studied animals (vertebrates; amphibians) there are likely still undiscovered species (18, 21, 22, 23, 24). This means that the number of undescribed species may be even higher for unexplored or inaccessible areas or taxa[1].
This also means that we in most cases know little about their roles in the ecosystem, how they interact with other species, and how the extinction may affect the ecosystem as whole.
Describing species takes a long time, averagely 21 years! (20). Much due to the shortage of taxonomists and that most species are rare and only represented by single specimens, leading to many researchers waiting until they have more specimens available[2] (20, 25). Museum collections are reservoirs of many undescribed species, sampled in bulk on long ago expeditions and many of which may already be extinct (20). Another factor is that most taxonomists don’t live in developing countries where Biodiversity is highest (20, 26). Many species can also be relatively well known by those who do live there (23), but have never been formally described or studied in other contexts. If no one has studied a species, how can we know if it is a common, widespread species or a group of similar endemic species? This is especially problematic in cases where their most well-known quality is that they are especially tasty with soy sauce (27*[3]).
There is an additional dimension to this, what about the already described species? Does everybody agree on what a species is? (This is even further complicated by species concepts).
The problem is how we define what a species is, the method used, and the disagreement between these. Many taxonomists and sytematicists rely either on morphology or DNA to define species. Either they don’t trust one method, or they don’t have a choice, like paleontologists (28) or those researching cryptic species[4] (29).
However, In some cases convergent evolution[5] can make unrelated taxa that have some habits or habitats in common to seem closely related by morphology (30, 31) or populations that seem identical may in fact be evolutionary distinct (32) This may lead to conflict between methods and lead to bad taxonomy in the form of wrongful synonyms[6] (many taxa lumped into one) or undiscovered synonyms (one taxa counted as several). Mora et al. (18) asked 2938 taxonomists about what they felt could limit taxonomic effort[7], most answered synonyms as a major problem on the species level even if did not affect higher taxonomic levels drastically. So likely the general trends in taxa response to stressors can be trusted, but on the species level this resolution may be lacking.
How to define speices can wary form disiplines to the methods used within the disiplines.
This can be highly problematic in conservation of Biodiversity and ecosystem services. A study by Prie et al. (33) on the freshwater clams Unio in Europe, found three distinct species and several distinct sub species based on DNA. This group is riddled with synonyms based on morphology, which has impacted the conservation status of the species, leading to some of the groups to be isolated and population reduced due to human activities (33).
This lack of knowledge is dangerous as it reduces our ability to conserve species or communities that may be potentially important for ecosystem function (24, 33, 34, 35). Additionally, if enough species are not properly recognized we cannot see the complete picture of biodiversity patterns or estimate extinction rates (21, 24). It also makes it difficult to transform the scientific data into information that can be used by legislators who decide on conservation and the people who actually live alongside the species (however some manage *).
We stand to lose important ecosystem processes and services that may in turn harm other species, or even come to affect human populations (21, 34, 36, 37). Basically we don’t really know what we have lost until it is gone. Due to this it would beneficial not only to protect areas that we know to be especially biodiverse, but also areas that are relatively unexplored or less disturbed by humans, as there are likely a high diversity of undescribed species there (24).
References
- Wilson, E. O. (1988). The current state of biological diversity. In Biodiversity, E.O. Wilson & F.M. Peters, (Eds). 3-18 PP. Washington, DC: National Academy Press. Accessed at http://www.ncbi.nlm.nih.gov/books/NBK219273/ at 20.02.16
- Walther, G. R., Post, E., Convey, P., Menzel, A., Parmesan, C., Beebee, T. J., et al., & Bairlein, F. (2002). Ecological responses to recent climate change. Nature, 416(6879), 389-395.
- Walther, G. R. (2010). Community and ecosystem responses to recent climate change. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 365(1549), 2019-2024.
- Parmesan, C. (2006). Ecological and evolutionary responses to recent climate change. Annual Review of Ecology, Evolution, and Systematics, 637-669.
- Alongi, D. M. (2002). Present state and future of the world’s mangrove forests. Environmental conservation, 29(03), 331-349.
- Stuart, S. N., Chanson, J. S., Cox, N. A., Young, B. E., Rodrigues, A. S., Fischman, D. L., & Waller, R. W. (2004). Status and trends of amphibian declines and extinctions worldwide. Science, 306(5702), 1783-1786.
- Brook, B. W., Sodhi, N. S., & Bradshaw, C. J. (2008). Synergies among extinction drivers under global change. Trends in Ecology & Evolution, 23(8), 453-460
- Butchart, S. H., Walpole, M., Collen, B., Van Strien, A., Scharlemann, J. P., Almond, R. E, et al. & Carpenter, K. E. (2010). Global biodiversity: indicators of recent declines. Science, 328(5982), 1164-1168.
- Polidoro, B. A., Carpenter, K. E., Collins, L., Duke, N. C., Ellison, A. M., Ellison, J. C., et al. & Livingstone, S. R. (2010). The loss of species: mangrove extinction risk and geographic areas of global concern. PloS one, 5(4), e10095.
- Vitousek, P. M., Mooney, H. A., Lubchenco, J., & Melillo, J. M. (1997). Human domination of Earth’s ecosystems. Science, 277(5325), 494-499.
- Chapin III, F. S., Zavaleta, E. S., Eviner, V. T., Naylor, R. L., Vitousek, P. M., Reynolds, H. L., et al. & Mack, M. C. (2000). Consequences of changing biodiversity. Nature, 405(6783), 234-242.
- Van der Putten, W. H., Macel, M., & Visser, M. E. (2010). Predicting species distribution and abundance responses to climate change: why it is essential to include biotic interactions across trophic levels. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 365(1549), 2025-2034.
- Stork, N. E. (1993). How many species are there?. Biodiversity & Conservation, 2(3), 215-232.
- Blois, J. L., Zarnetske, P. L., Fitzpatrick, M. C., & Finnegan, S. (2013). Climate change and the past, present, and future of biotic interactions. Science, 341(6145), 499-504.
- Raup, D. M. Taxonomic diversity during the Phanerozoic. Science 177, 1065–1071 (1972).
- Benton, M. J., Wills, M. A., & Hitchin, R. (2000). Quality of the fossil record through time. Nature, 403(6769), 534-537.
- Chapman, A. D. Numbers of living species in Australia and the world. (2009): 1-78 PP. Report for the Australian Biological Resources Study. Canberra, Australia. Available form https://www.environment.gov.au/system/files/pages/2ee3f4a1-f130-465b-9c7a-79373680a067/files/nlsaw-2nd-complete.pdf accessed on 20.02.16.
- Mora, C., Tittensor, D. P., Adl, S., Simpson, A. G., & Worm, B. (2011). How many species are there on Earth and in the ocean?. PLoS Biol, 9(8), e1001127.
- May, R. M. (2011). Why worry about how many species and their loss?. PLoS Biol, 9(8), e1001130.
- Fontaine, B., Perrard, A., & Bouchet, P. (2012). 21 years of shelf life between discovery and description of new species. Current Biology, 22(22), R943-R944.
- Ceballos, G., & Ehrlich, P. R. (2009). Discoveries of new mammal species and their implications for conservation and ecosystem services. Proceedings of the National Academy of Sciences, 106(10), 3841-3846.
- Geissmann, T., Lwin, N., Aung, S. S., Aung, T. N., Aung, Z. M., Hla, T. H., Grindley, M., Momberg, F. (2011). A new species of snub‐nosed monkey, Genus Rhinopithecus Milne‐Edwards, 1872 (Primates, Colobinae), from northern Kachin State, northeastern Myanmar. American Journal of Primatology, 73(1), 96-107.
- Hart, J. A., Detwiler, K. M., Gilbert, C. C., Burrell, A. S., Fuller, J. L., Emetshu, M., Hart, T. B., Vosper, A., Sargis, E. J, Tosi, A. J. (2012). Lesula: a new species of Cercopithecus monkey endemic to the Democratic Republic of Congo and implications for conservation of Congo’s Central Basin. PLoS One, 7(9), e44271.
- Giam, X., Scheffers, B. R., Sodhi, N. S., Wilcove, D. S., Ceballos, G., & Ehrlich, P. R. (2012). Reservoirs of richness: least disturbed tropical forests are centres of undescribed species diversity. Proceedings of the Royal Society of London B: Biological Sciences, 279(1726), 67-76.
- Lim, G. S., Balke, M., & Meier, R. (2012). Determining species boundaries in a world full of rarity: singletons, species delimitation methods. Systematic biology, 61(1), 165-169.
- Gaston, K. J., May, R. M.(1992). Taxonomy of taxonomists. Nature, 356, 281-282.
- Lim K. K. P., Murphy D. H, Morgany, T., Sivasothi, N.,Ng P. K. L. Soong, B. C.,. Tan, H. T. W, Tan, K. S., Tan, T. K. A (1999) Guide to Mangroves of Singapore Peter K. L. Ng and N. Sivasothi (ed). BP Guide to Nature Series published by the Singapore Science Centre, sponsored by British Petroleum. Raffles Museum of Biodiversity Research, The National University of Singapore & The Singapore Science Centre. Online excerpt: http://mangrove.nus.edu.sg/guidebooks/text/2088.htm accessed on 19.02.16
- Kaiser, G., Watanabe, J., & Johns, M. (2015). A new member of the family Plotopteridae (Aves) from the late Oligocene of British Columbia, Canada. Palaeontologia Electronica, 18(3), 1-18
- Jörger, K. M., & Schrödl, M. (2013). How to describe a cryptic species? Practical challenges of molecular taxonomy. Frontiers in Zoology, 10(1), 1.
- May, R. M. (1990). Taxonomy as destiny. Nature, 347(6289), 129-130.
- Oskars, T. R., Bouchet, P., & Malaquias, M. A. E. (2015). A new phylogeny of the Cephalaspidea (Gastropoda: Heterobranchia) based on expanded taxon sampling and gene markers. Molecular phylogenetics and evolution, 89, 130-150.
- Hebert, P. D., Penton, E. H., Burns, J. M., Janzen, D. H., & Hallwachs, W. (2004). Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proceedings of the National Academy of Sciences of the United States of America, 101(41), 14812-14817.
- Prié, V., Puillandre, N., & Bouchet, P. (2012). Bad taxonomy can kill: molecular reevaluation of Unio mancus Lamarck, 1819 (Bivalvia: Unionidae) and its accepted subspecies. Knowledge and Management of Aquatic Ecosystems, (405), 08.
- Suzán, G., Marcé, E., Giermakowski, J. T., Mills, J. N., Ceballos, G., Ostfeld, R. S., Armien, B,. Pascale, J. M.,Yates, T. L. (2009). Experimental evidence for reduced rodent diversity causing increased hantavirus prevalence. PloS one, 4(5), e5461
- Mace, G. M. (2004). The role of taxonomy in species conservation. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 359(1444), 711-719.
- Worm, B., Barbier, E. B., Beaumont, N., Duffy, J. E., Folke, C., Halpern, B. S.,. Jackson J. B. C, Lotze, H. K., Micheli, F., Palumbi, S. R., Sala, E., Selkoe, K. A., Stachowicz, J. J., Watsom, R. (2006). Impacts of biodiversity loss on ocean ecosystem services. Science, 314(5800), 787-790.
- Dugan, P. J., Barlow, C., Agostinho, A. A., Baran, E., Cada, G. F., Chen, D., et al. & Marmulla, G. (2010). Fish migration, dams, and loss of ecosystem services in the Mekong basin. Ambio, 39(4), 344-348.
[1] Taxa- A group of individuals or species that due to one or several methods are seen my Taxonomists or sytematicists to form one discernable unit and given a name.
[2] I have more than once spent two whole days dissecting a specimen less than 1 mm long as it was the only one available.
[3] I am revising the taxonomy of this group, current knowledge is next to none.
[4] Cryptic species- A species that is molecular distinct, but more or less impossible to separate form related species based on morphology.
[5] Convergent evolution- When evolution drives two species that are unrelated to gain a similar morphology, for example due to living in a similar habitat, also possible for related, but not closely related species trough parallel evolution.
[6] Synonymy- Basically when one taxa is regarded as the same as another and the most recent name must be replaced by the oldest available name.
[7] Taxonomic effort- Species discovery rates and to some degree the number of taxonomist working on a group (18).